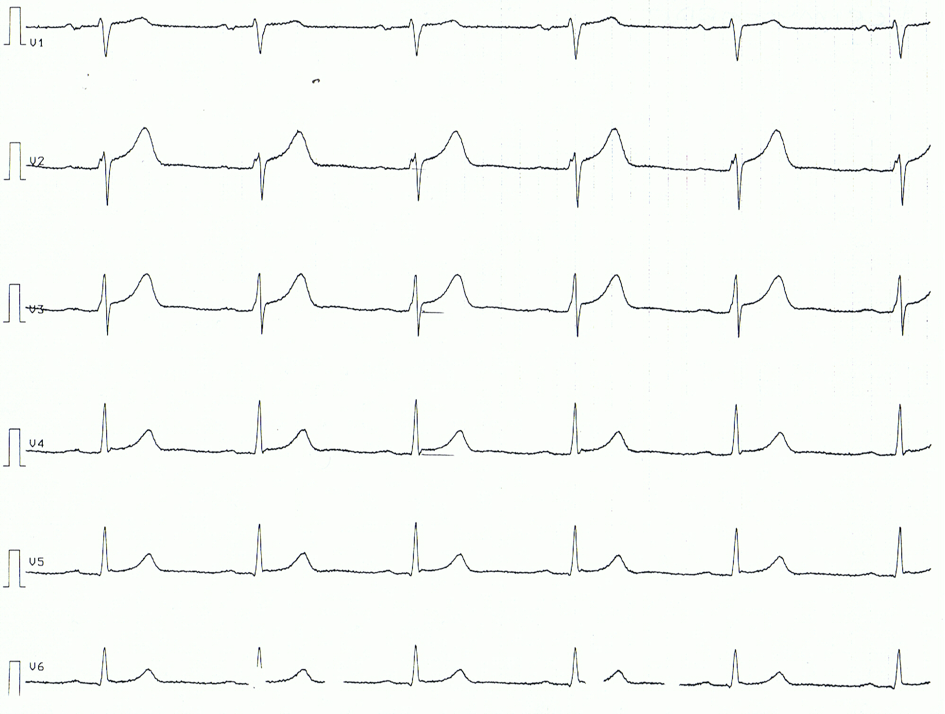
Trials of coronavirus disease 2019 (COVID-19) vaccination included limited numbers of children, so they may not have detected rare but important adverse events in this population. We report 7 cases of acute myocarditis or myopericarditis in healthy male adolescents who presented with chest pain all within 4 days after the second dose of Pfizer-BioNTech COVID-19 vaccination. Acute COVID-19 was ruled out in all 7 cases on the basis of negative severe acute respiratory syndrome coronavirus 2 real-time reverse transcription polymerase chain reaction test results of specimens obtained by using nasopharyngeal swabs. None of the patients met criteria for multisystem inflammatory syndrome in children. Six of the 7 patients had negative severe acute respiratory syndrome coronavirus 2 nucleocapsid antibody assay results, suggesting no previous infection. Cardiac MRI revealed late gadolinium enhancement characteristic of myocarditis.
Three patients were treated with nonsteroidal antiinflammatory drugs only, and 4 received intravenous immunoglobulin and corticosteroids. In this report, we provide a summary of each adolescent's clinical course and evaluation. No causal relationship between vaccine administration and myocarditis has been established. Continued monitoring and reporting to the US Food and Drug Administration Vaccine Adverse Event Reporting System is strongly recommended.
"The American Heart Associationrecommends all health care professionals be aware of these very rare adverse events that may be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, or symptoms of severe inflammation. Health care professionals should strongly consider inquiring about the timing of any recent COVID vaccination among patients presenting with these conditions, as needed, in order to provide appropriate treatment quickly. As detailed in last month's AHA/ASA statement, all suspected CVST or blood clots associated with the COVID-19 vaccine should be treated initially using non-heparin anticoagulants.
Heparin products should not be administered in any dose if TTS/VITT is suspected, until appropriate testing can be done to exclude heparin-induced antibodies. In addition, health care professionals are required to report suspected vaccine-related adverse events to the Vaccine Adverse Event Reporting System, in accordance with federal regulations. Cases of myocarditis and/or pericarditis following immunization with COVID-19 vaccines have been reported in a small number of people in Canada and internationally.
Health Canada and other international regulators are continuing to investigate the potential relationship between COVID-19 vaccines and these rare events. Most reported cases to date have followed vaccination with an mRNA vaccine (Pfizer-BioNTech and Moderna) and, based on an analysis of international cases, have occurred more often after the second dose and in younger male adults and adolescents. The Canadian evidence is expected to evolve as more people in these populations are vaccinated. Available short-term follow-up data show that these events were typically mild and treatable; however, information on long-term outcomes is not yet available. In contrast, chest pain, irregular heartbeat, heart palpitations, shortness of breath and light-headedness could indicate myocarditis or pericarditis. Symptoms of these conditions have generally occurred within seven days of vaccination.
The Commission on Human Medicines has reviewed the available safety data and advises healthcare professionals to be alert to the signs and symptoms of myocarditis and pericarditis. Vaccinated individuals should be advised to seek immediate medical attention should they experience new onset of chest pain, shortness of breath or symptoms of arrhythmia. As of May 12, 2021, children in the United States aged ≥12 years are now eligible to receive the Pfizer-BioNTech vaccine. Primary care and ED physicians and health care providers should consider myocarditis an etiology of chest pain in patients with recent COVID-19 mRNA vaccination.
Elevated serum troponin, an abnormal ECG, and an abnormal cardiac MRI were seen in all cases . "We remain confident that the benefits of vaccination far exceed the very small, rare risks. The risks of vaccination are also far smaller than the risks of COVID-19 infection itself, including its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including myocarditis. Myocarditis and pericarditis are diseases that cause inflammation of the heart that can occur following infections or immune-related diseases. Symptoms of myocarditis and pericarditis can vary but may include shortness of breath, palpitations, irregular heartbeats, and chest pain.
The public is advised to seek medical attention if they experience any of these symptoms following vaccination with Covid-19 vaccines. While cases of myocarditis and pericarditis linked to Covid vaccination are rare, they are likely underreported, the researchers said, pointing to a recent report by the CDC suggesting an incidence of about 4.8 cases of myocarditis per one million vaccinated individuals. "This study shows a similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting," they wrote. Additionally, pericarditis may be more common than myocarditis among older patients. The known and potential benefits of COVID-19 vaccination outweigh the known and potential risks, including the possible risk of myocarditis or pericarditis.
Also, most patients with myocarditis and pericarditis who received care responded well to treatment and rest and quickly felt better. More than 800 unconfirmed cases of heart problems were reported to the vaccine safety monitoring system, but the CDC says it's most concerned about people under age 30. In that age group, the agency says it saw higher-than-usual numbers of myocarditis and pericarditis, with 226 confirmed cases. The CDC advisory committee will get an update on those reports at the meeting next week. Severity of myocarditis and pericarditis cases can vary, but "reports have increased since April, mostly in young males 16 and older, several days after vaccination, and more often after the second vaccine dose.
Clinicians should be aware of the risk of myocarditis and pericarditis with mRNA vaccines and those most likely to be affected. They should be alert to presentations such as acute chest pain, shortness of breath and palpitations that may be suggestive of myocarditis after vaccination, especially in adolescent or young males. Coronary events are less likely to be the source of such symptoms among younger people.
A strong signal of myocarditis/pericarditis has been reported recently with mRNA COVID-19 vaccines in the United States . However, the US Advisory Committee on Immunization Practices has concluded that the benefits of mRNA COVID-19 vaccines continue to outweigh the risks of myocarditis and pericarditis even among young people. For persons over 30 years of age, the reporting rates were 2.4 and 1.0 per million second doses, respectively, for males and females. Currently, about 1,000 cases of myocarditis and pericarditis have been reported after vaccination against COVID-19 with one of the mRNA vaccines, Pfizer/BioNTech or Moderna. The cases have been most common in male adolescents and young adults, occurring most often after the second dose, and usually within several days of receiving the vaccine.
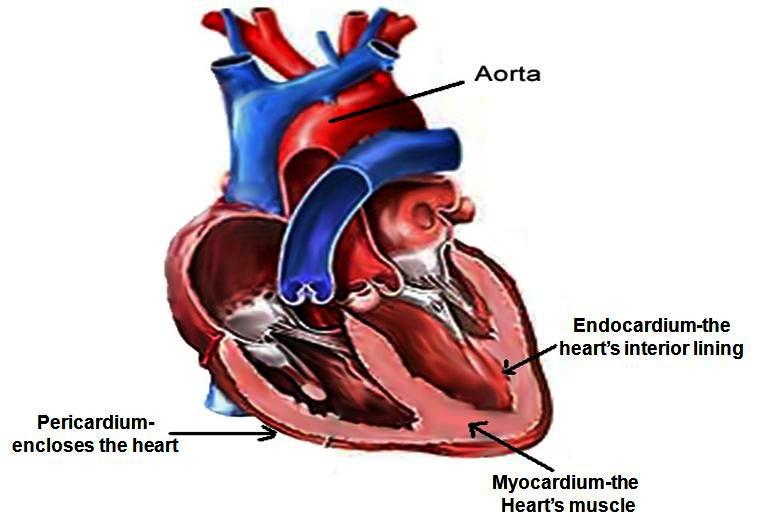
Experts are still gathering information, but as of this writing, 79% of teens and young adults who experienced this had recovered. The EMA encourages all healthcare professionals to report any cases of myocarditis or pericarditis and other adverse events in people receiving COVID-19 vaccines. As the country continues to push for more people to get vaccinated against COVID-19, some remain concerned over rare cases of heart inflammation—myocarditis and pericarditis—linked to the Pfizer-BioNTech and Moderna mRNA vaccines. While some parents may be thinking twice about teen vaccination, medical experts reassure that the risk of myocarditis and pericarditis are far lower than the risks of serious illness or death from contracting COVID-19. Be alert to the signs and symptoms of myocarditis and pericarditis when individuals present with chest pain, shortness of breath, palpitations or other signs and symptoms following immunization with a COVID-19 vaccine. Myocarditis is an inflammation of the heart muscle, while pericarditis is an inflammation of the lining around the heart.
Both conditions can result from an infection (including COVID-19), exposure to a toxic substance or radiation, or other health events. Symptoms can include chest pain, shortness of breath, or palpitations (feelings of having a fast-beating, fluttering or pounding heart). In many cases, these conditions are mild and require little to no treatment. More data have become available since the GACVS statement of 26 May 2021, with more countries reporting myocarditis and pericarditis in individuals who received COVID-19 mRNA vaccines. The reported cases have typically occurred within days of vaccination, more commonly among younger males and more often following the second dose the of COVID-19 mRNA vaccines. In their review of the Pfizer-BioNTech vaccine, regulators paid close attention to an American health care claims database, which found that the risk of the conditions in 16- and 17-year-old vaccinated boys could be as high as 1 in 5,000.
The cases in the database were unconfirmed, the F.D.A. cautioned in an analysis published this week, but they were considered a reasonable estimate of the possible risk. Even in the worst-case scenarios of post-vaccination myocarditis and pericarditis modeled by the F.D.A., the benefits of vaccination still outweighed the risks, the analysis said. Reporting rates were 40.6 cases per million second doses of mRNA COVID-19 vaccines for males aged 12 to 29 years and 2.4 per million second doses for males aged 30 or older.
For females their reported rates were 4.2 and 1.0 per million second doses in these age groups and the highest reporting rates were among males aged 12 to 17 and those aged 18 to 24 years. In Israel, 27 of the 148 cases occurred around receipt of the first vaccine dose and 121 occurred within 30 days after the second vaccine, with most cases in men aged 16 to 19 years. Presentation of acute myocarditis is variable, ranging from subclinical disease to heart failure and patients can present with chest pain, shortness of breath, palpitations and fatigue.
Most patients respond well to standard treatment, and the prognosis is good. However, it can progress to dilated cardiomyopathy and chronic heart failure, with evidence implicating it in 12% of sudden deaths in adults aged under 40. While myocarditis has not been officially listed as a rare adverse event related to mRNA-based COVID-19 vaccines, an editorial related to both reports lines up the circumstantial evidence. Although five people died, the review stated that they were all either elderly or had underlying health conditions.
"These reports are extremely rare, and the events are typically mild with individuals usually recovering within a short time with standard treatment and rest," it said. Confirmed cases have occurred mostly in male adolescents and young adults aged 16 years or older. But given the hundreds of millions of vaccine doses administered, says the CDC, reports of myocarditis and pericarditis are rare. While acknowledging the clear benefits of the mRNA vaccines in reducing deaths and hospitalizations due to COVID-19 infections, the subcommittee encourages all health professionals to report all events of myocarditis and other adverse events observed with these and other vaccines.
The WHO COVID-19 vaccine safety surveillance manual provides guidance to countries on the safety monitoring and adverse events data sharing for the new COVID-19 vaccines. Extensive diagnostic evaluation for other myocarditis etiologies was negative , including respiratory pathogens from nasopharyngeal swabs, serum PCR tests, and infectious serologies. Meanwhile, health experts are concerned that even rare reports of myocarditis, like previously reported adverse effects associated with COVID vaccines, will increase vaccine hesitancy.
And the overwhelming consensus is that the benefits of COVID-19 vaccinations still far outweigh the low risk of developing the heart condition. The VSD is another safety monitoring system that reported an increased incidence of heart inflammation in people between 16 and 34 years old after their second vaccine dose compared to the rate seen after the first dose. There were 285 observed cases of heart inflammation after the second dose in those between 16 and 24 years old in the VAERS data.
The expected cases were 10 to 102 cases for the age bracket, based on U.S. population background incidence rates. The 23 US military men all received mRNA-based COVID-19 vaccines from Jan 1 to Apr 30, with 16 receiving Moderna and 7 receiving Pfizer 4 days prior to symptom onset. All had severe chest pain as well as 10- to 400-fold increased cardiac troponin levels, which indicates heart damage. Three had been infected with COVID-19 prior to vaccination and had symptoms after their first dose, while the rest had symptoms after their second.
None of the patients had findings consistent with infectious, ischemic, or autoimmune etiologies. Pericarditis is a condition often related to myocarditis, and involves swelling and inflammation of the pericardium, a sac-like structure with two thin layers of tissue that surround the heart to hold it in place and help it function properly. Symptoms of pericarditis are sharp, stabbing chest pain that comes on suddenly; fever; weakness; and trouble breathing or shortness of breath. Approximately 45,000 people in the U.S. are hospitalized with pericarditis each year. Myocarditis is inflammation of the middle layer of the wall of the heart muscle, and it can weaken the heart muscle and the heart's electrical system, which keeps the heart pumping regularly. Severe myocarditis can lead to heart failure, abnormal heartbeat and sudden death.
Signs and symptoms of myocarditis are chest pain, abnormal heartbeat and unexpected shortness of breath. Approximately 10 to 20 per 100,000 people are diagnosed with myocarditis in the U.S. annually, and many cases resolve on their own or with treatment, leading to a full recovery. The few cases of myocarditis that have been reported after COVID-19 vaccination are being investigated. However, myocarditis is usually the result of a viral infection, and it is yet to be determined if these cases have any correlation to receiving a COVID-19 vaccine, especially since the COVID-19 vaccines authorized in the U.S. do not contain any live virus. In addition to chest pain, other possible symptoms of myocarditis or pericarditis include shortness of breath or unusual heart rhythms. "If you have any such symptoms after getting the Covid-19 vaccine notify your doctor," Wong advised.
"It may not be the result of the vaccine and other things may be going on," so it is important to "be aware of one's self" and to have your doctor to assess your situation. The agency advises seeking medical attention if you have chest pain, shortness of breath, or feelings of fast-beating, fluttering or pounding heart after receiving either vaccine. These warnings were added in late June when heart inflammation occurrences were first linked to the mRNA vaccines. Now health officials arelookinginto Canadian data that suggests the risk of heart inflammation after Moderna's shot is occurring at higher rates in younger adults than previously believed.
The Post quoted a source saying the myocarditis risk could be 2.5 times higher for those who receive the Moderna vaccine over Pfizer's shot. Males under 30appearto be the most at risk of developing the side effect with either vaccination. Vaccinated individuals should be instructed to seek immediate medical attention if they develop symptoms indicative of myocarditis or pericarditis such as new onset and persisting chest pain, shortness of breath, or palpitations following vaccination. A risk of myocarditis and pericarditis has been observed in people who have received mRNA COVID-19 vaccines in overseas studies, particularly in males under 30 years of age after the second vaccine dose. An increased risk of heart inflammation has been observed in people who have received mRNA COVID-19 vaccines in overseas studies, particularly in males under 30 years of age after the second vaccine dose. Cases of myocarditis and pericarditis have been reported in the United States and other countries after Comirnaty® administration.
These case reports were more common in adolescents and young adults, and after administration of the second vaccine dose. The companies are studying lower dosing in children to alleviate some of the risk. In the U.S., cases have mostly been among male adolescents and young adults under 30. Most patients who received care responded well to treatment and rest and made a full recovery.
The National Advisory Committee on Immunization is recommending informed consent for people receiving an mRNA vaccine should include a discussion about the very rare risk of myocarditis and/or pericarditis following immunization. As a precaution, NACI recommends that individuals who experienced myocarditis and/or pericarditis after a first dose of an mRNA vaccine should wait to get their second dose until more information is available. Study limitations include cases missed in outside care settings and missed diagnoses of myocarditis or pericarditis , as well as inaccurate EMR vaccination information. Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship. Hopefully, these hospital consortium investigators will expand their analysis to compare other events in the vaccinated and unvaccinated populations and in COVID-19 patients. Most pre-existing cardiac conditions are not regarded as contraindications to vaccination.
However, people with inflammatory or unstable heart disease especially should be advised about symptoms to be aware of and to seek medical care if they develop them or have worsening of pre-existing symptoms. A precautionary review in the days after their vaccination may also be advised. IMAC emphasises that the overwhelming benefits of vaccination in protecting against COVID-19 greatly outweigh the rare risk of these conditions, and Comirnaty continues to be recommended for all people ≥ 16 years of age who do not have any contraindications to the vaccine. Recent data from Israel showed three excess cases of myocarditis per 100,000 following Comirnaty vaccination versus 11 excess per 100,000 with COVID-19 infection.
Myocarditis symptoms such as chest pain, shortness of breath or feelings of having a fast-beating fluttering, or pounding heart may appear within a few days of having the vaccine. Anyone who experiences these symptoms after having the vaccine should seek medical attention. Symptoms of myocarditis can include new onset chest pain, shortness of breath and an abnormal heartbeat. It's important that anyone who experiences these symptoms in the first few days after vaccination seeks medical attention promptly.
While the incidence was higher than previously reported by the CDC, Diaz said that might be the result of actively searching for cases in the EMRs as opposed to voluntary reporting in VAERS. Additionally, one of the strengths of their data is that researchers were able to tease out myocarditis and pericarditis separately based on the patient charts, something that isn't always possible in adverse event reporting. Four patients received IVIg and oral prednisone; 1 of these 4 patients also initially received high-dose methylprednisolone . It is unclear if treatment with IVIg and/or corticosteroids, in the absence of MIS-C criteria, is warranted with all cases of myocarditis that develop temporally after COVID-19 vaccination. In the first few days after your vaccination seek medical attention if you experience new onset chest pain, shortness of breath or an abnormal heartbeat.
The case rate of myocarditis potentially connected to COVID vaccinations is still below the background rate in that age group from other causes, said Dr. Genevieve Buser, a pediatric infectious disease specialist at Providence St. Vincent. Moreover, the reported cases have generally been mild, and resolved quickly with chest pain going away and heart function returning to normal with the aid of Ibuprofen and other anti-inflammatories, she said. Hospital stays have been short and there don't seem to be any other lingering effects. Some 6.4 million adolescents aged 12 to 17 in the United States have received at least one dose of COVID-19 vaccine, according to data from the Centers for Disease Control and Prevention. And since April, there have been increased reports from Israel, Canada and the United States of inflammation of the heart happening after immunization mostly in that age group.